ALUMINITE
Class : Sulphates, chromates, molybdates
Subclass : Hydrated sulfates
Crystal System : Monoclinic
Chemistry : Al2(SO4)(OH)4 7H2O
Rarity : Uncommon
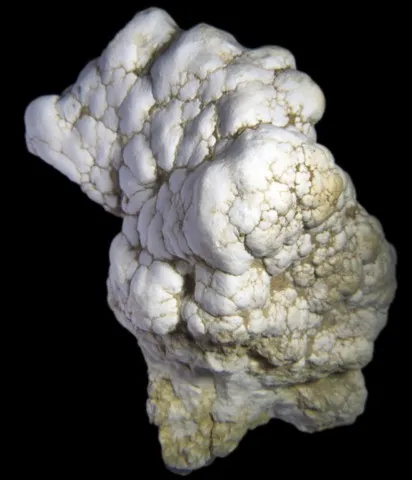
Aluminite is a recently formed hydrated aluminum sulphate observed in clayey environments, in maerls and lignites. It is an infrequent mineral which is formed by the action of sulphated solutions deriving from the oxidation of sulfides (pyrite) on alumina silicates. It is its name to its chemical composition. It presents as reniform or nodular masses, sometimes spherolitic, made up of tiny fibers, white to pale greyish-green or pale pink-orange in color.
Main photo : Aluminite from Newhaven Cliffs, England © Vik Vanrusselt
Aluminite in the World
Twinning
No twin known for this mineral species.
Fakes and treatments
No fakes reported for this mineral species.
Hardness : 1 to 2
Density : 1,7
Fracture : Irregular
Trace : White
TP : Translucent to opaque
RI : 1.459 to 1.470
Birefringence : 0,011
Optical character : Biaxial +
Pleochroism : None
Fluorescence : None
Solubility : Nitric acid and hydrochloric acid
Magnetism : None
Radioactivity : None