Fluorescence : How to choose your UV lamp
What are UV (or ultraviolet) ?
The light our eyes respond to is a form of energy that moves in waves. Our eyes see it as white light, but it's actually a combination of red, orange, yellow, green, blue, and purple spectral colors.
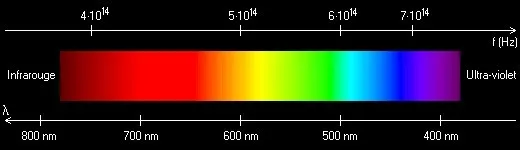
Visible light is a very small part of what scientists call the electromagnetic spectrum, which also includes high-energy invisible rays like gamma rays and x-rays, and low-energy rays like radio waves. Visible light is near the middle of the electromagnetic spectrum.
The wavelengths of the electromagnetic spectrum are measured in nanometers (nm). The visible spectrum starts at around 400 nm (purple) and extends to around 700 nm (red). Our eyes cannot see energy waves outside of this range. The shorter the wavelength, the higher the energy. Rays with shorter wavelengths and higher energies than violet constitute ultraviolet (UV) radiation. UV radiation ranges from about 200 nm to 400 nm in the electromagnetic spectrum.
UV radiation is invisible, but when it interacts with certain minerals, it emits visible light. A general term for this light emission is "luminescence". X-rays can also cause the luminescence of minerals, but this can only be done in the laboratory, this is called X-ray fluorescence spectroscopy analysis.
The two main types of luminescence that occur in minerals are fluorescence and phosphorescence. The fluorescence lasts as long as the stone is stimulated by the radiation. With phosphorescence, the stone continues to fluoresce once the radiation source is removed. Fluorescence is much more common than phosphorescence.
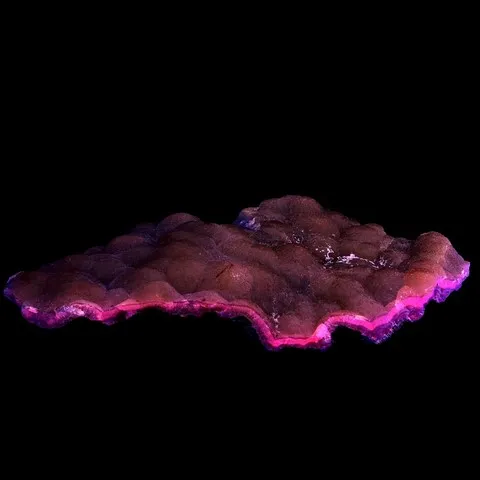
Photo : Petroleum inclusions in quartz fluorescent under long wave UV light (Pakistan)
Why the fluorescence ?
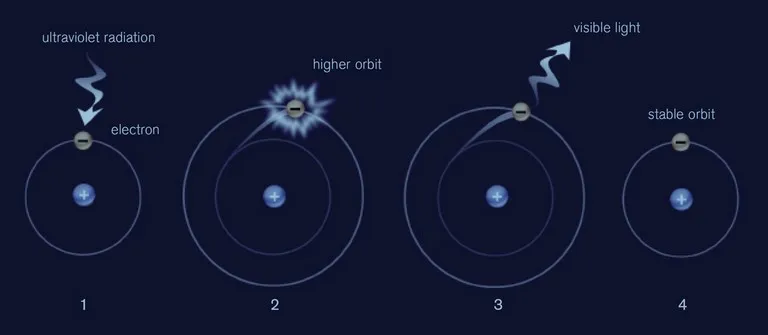
Electrons in transition elements like cobalt and chromium absorb UV rays. When electrons absorb energy, they find themselves in an "excited" state and they must release this energy to return to their normal state. Most often they transfer it to the crystal in the form of heat. Sometimes, however, electrons convert some of the energy into visible light, creating fluorescence. Certain chemical elements inhibit fluorescence. For example, if iron is part of the crystal structure of a mineral or is present as an impurity, it will reduce fluorescence or eliminate it altogether. Certain minerals with significant iron content are inert to UV rays such as orange to red garnets, demantoid and andradite garnet, peridot, aquamarine, iolite, nephrite or citrine. Many Thai rubies are high in iron, so they aren't as fluorescent as rubies from other regions - like Myanmar and Vietnam - which contain much less iron. Verneuil synthetic rubies have a higher chromium content than natural stones and contain almost no iron, so they exhibit extremely strong and luminous reactions to UV. Most synthetic emeralds are high in chromium and iron, so they often exhibit a red glow similar to the reaction of synthetic ruby. But some hydrothermal synthetic emeralds produced in Russia contain enough iron to inhibit fluorescence, fluorescence alone does not always allow conclusions to be drawn.
Because the fluorescence of a mineral is so closely related to the presence of certain trace elements in its chemical composition, many crystals of the same species or variety exhibit widely varying reactions to UV radiation.
The different types of UV
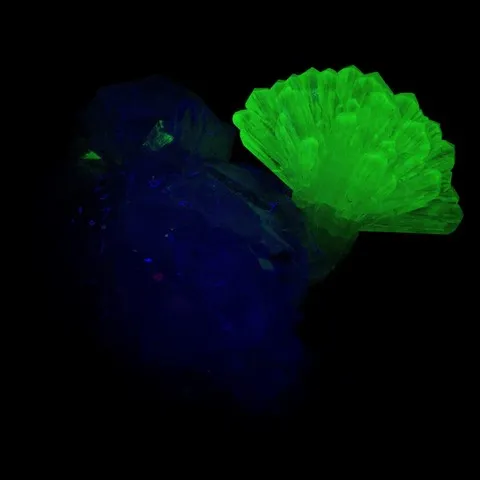
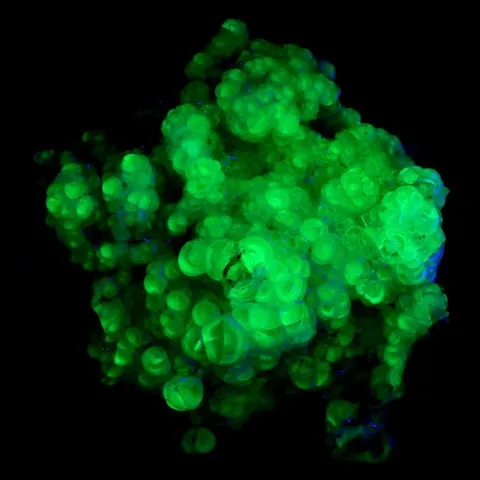
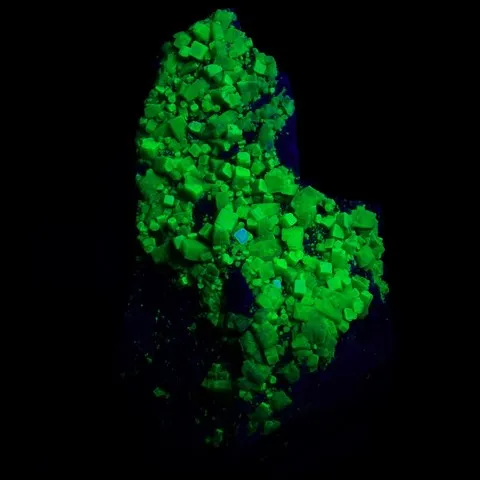
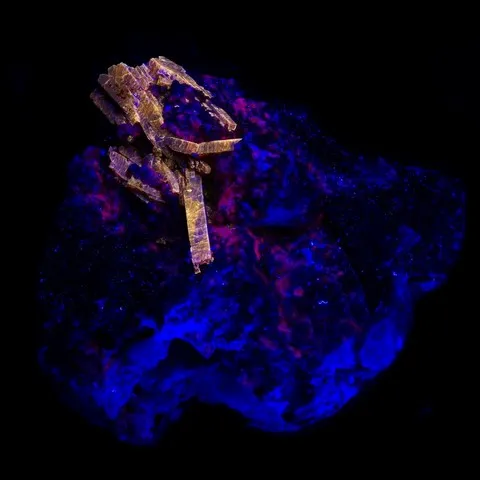
Fluorite from Madagascar fluorescent under long wave UV
Smithsonite from Tsumeb fluorescent under short wave UV
Autunite from Les Oudots fluorescent under long & short wave UV light
Zircon & scapolite of Madagascar under short wave UV light
The different types of UV lamps



Our advice in choosing a UV lamp
The wattage or number of LEDs : The most important parameter to look at when buying a UV lamp is its wattage (W). The more powerful a UV lamp, the stronger and more easily observable the luminescence (but the more its price will be as high). In general, a 9W tube lamp requires use in the dark in order to observe fluorescence, while from 36W fluorescence is observed in daylight... For LED torches the determining technical factor is the number of LEDs, the more there are, the more powerful the lighting will be.
The quality of UV filtration : The filter is an essential part of UV lamps which aims to allow only one frequency of waves to pass (254 nm, 312 nm or 368 nm), their thickness is variable. It is also the most expensive part. However, without this filter the fluorescence is not observable correctly, if not observable at all. A lamp fitted with a good filter does not leave the bulb visible. For LED torches this criterion is not decisive, the emission spectrum of a UV LED being much more restricted than that of a bulb.
The type of waves : the type of wave remains a personal choice, the ideal is of course to have both short wave UV and long wave UV. Short wave UV is more expensive than long UV but also reacts more with many mineral species. The average UV is of no real interest and is even more expensive than the short UV.
Our choice
The choice that seems the most judicious to us, if you want to be interested in fluorescence and initially opt for a long wave UV LED torch (390-410 nm) for its portability, its power and its low price. It allows you to really discover the world of fluorescence in a simple and straightforward way. If subsequently you are seduced by the small world of fluorescence, then we advise you to invest in a tube lamp operating on both short waveUV + long wave UV of at least 36W (Medium wave UV is not really interesting in our opinion). Be careful on the latter to check that the operating voltage is that of your country (230V for Europe; 110V for the USA).
Safety reminders
Be careful, even if fluorescence is fun, UV lamps are dangerous if they are not handled with care. They should not be used without the supervision and responsibility of an adult, they are not toys. UV radiation, if projected on the eyes, can cause permanent blindness, it is recommended to wear protective glasses when using. It can also cause severe burns to the skin with prolonged exposure (such as sunburn).