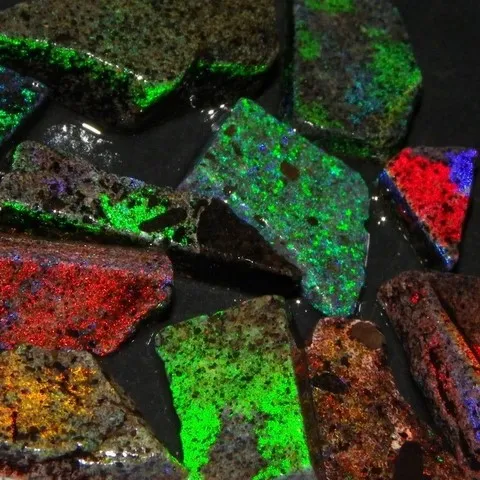OPAL
Class : Silicates
Subclass : Tectosilicates
Crystal system : Amorphous/Disordered
Chemistry : SiO2 nH2O
Rarity : Common
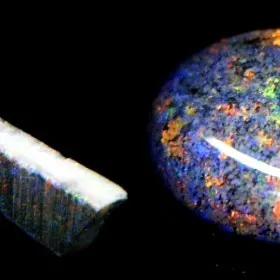
The opal is an amorphous silica gel or has a low disordered crystallization. Mostly made of silica microspheres (150 to 300 nm in diameter) visible only under the electron microscope and sometimes associated with disordered fibers of cristobalite and tridymite. In mineralogy, opals are classified into 3 families : opale-CT (silica gel + disordered fibers of cristobalite and tridymite), opal-C (silica gel + cristobalite fibers) and opal-A (silica gel only). The void between the microspheres are filled by air or water whose content can vary from 6% to more than 20%. The play-of-color (abusively called "fires") of certain opals results from the diffraction of the light through these layers of microspheres. The term "opal" comes from Sanskrit "upala" (precious stone) and then taken up by the Greek "opalios" and the Latin "opalus". It is generally found in volcanic and sedimentary environments. In volcanic areas, it is deposited at the hot water source and around geysers rich in silica (geyserite) or by circulation of hot siliceous waters affected by the fumarolic activity (gases). In sedimentary areas, it is formed by alteration of silicates and colloidal deposition (tiny particles of the size of the nanometer), in this case it sometimes happens to epigenize fossil organisms. Due to its partially totally amorphous nature, it never occurs in crystals, but in botryoidal or stalactitic masses, more rarely in veins. Finally, in a more anecdotal way, the opal constitutes the skeletons of some living beings : spicules of sponges, or shells of diatoms (siliceous algae) and radiolar (zooplankton) which by accumulation can give rocks (diatomite and radiolarite). The opal can be opaque to transparent and of varied colors resulting of many varieties : hyalite (transparent and colorless), cacholong (milky white), resinite (yellow to brown), fire opal (red-orange and transparent) ; The precious opals present a play-of-color and are also classified according to their colors and transparencies : black opal (black with play-of-color), crystal (transparent with play-of-color), boulder (with matrix), koroit (thin veins in matrix with violent play-of-color), chocolate (brown with play-of-color). It is a mineral which has a great importance in jewelery, but can also be used in industry, radiolarites and diatomites are crushed to make abrasives, filters and insulating and absorbent products, they also enter in the preparation of dynamite. Finally, diatomite is also widely used in the cinema industry because of its very light weight.
Opal in the World
Opal in France
France has some interesting opal deposits especially in Auvergne region correlate with the young volcanism : forcherite from St. Nectaire (yellow opal due to realgar and orpiment inclusions), resinite opal from Gergovie and the famous lussatite from Limagne. We also cano note the pink opal of Quincy curiosity in the Cher department locally named "quincyite" and whose color is due to the inclusions of colored sepiolites and organic pigments.
Special shapes
Opal can replace or mold the shape of some fossils : shells of bivalves and gastropods, bones, plants (petrified wood), belemnite rostres, ammonites, etc...
Treatments and synthetics
Due to its importance in jewelry, opals are usually treated but also synthesized, these practices are unfortunately not always disclosed on the market.
- The synthetic opals : sometimes called Gilson or also Kyocera, are opals are made by compression, the stratification of the silica microspheres is forced and gives an unatural columnar play-of-color. Under the microscope, the batches of silica microspheres give on the surface of the stone a snakeskin texture absolutely absent on the natural counterparts.
- Doublets or triplets : doublet if the cabochons are made of a thin layer of precious opal stuck on an opaque black base (basalt, glass, etc...) ; triplet if in addition the opal is covered with a layer of glass or colorless quartz.
- Treated black opals : often called Andamooka black opals or "sugar and smoke" these opals dipped in sugar syrup and then carbonized with sulfuric acid.
.
- Opalite : is found in tumbled stones, balls and beads for lithotherapy. It is just an industrial glass imitating opal, microscopic fluid texture, gas bubbles and refractive index different from the natural opal make it easy to make the difference.
- Slocum opal : also known as "slocum stone", doublet or triplet made of metal particles covered with a glass or quartz dome. Can also be made of metallic sheets directly included in a glass. These stones can only deceive the neophytes, the visual aspect is very different from natural opal.
- Crazing : natural phenomenon of rapid cracking (a few months) and fracturing of precious opals linked to dehydration. Some low-quality, low-cost precious opals with high porosity eventually suffer this phenomenon and break. In order to avoid unpleasant surprises, serious producers leave their rough opals in full sunlight a few months before cutting. It is impossible visually to know in advance if the opal pieces will undergo this phenomenon. To avoid : the black opals and chocolate opals from Ethiopia and Indonesia, the large pieces of rough coming from the countries considered as cutting centers (Brazil, Madagascar, India, etc...). Crazing can appear on every opal (ever the best quality) if it is heated.
Hardness : 5,5 to 6,5
Density : 1,9 to 2,3
Fracture : Irregular to conchoidal
Trace : White
TP : Opaque to transparent
IR : 1,40 to 1,46
Birefringence : -
Optical character : -
Pleochroism : None
Fluorescence : Yellow, green, blue
Solubility : Hydrofluoric acid
Magnetism : None
Radioactivity : None