Play-of-color in precious opals
Precious opals fascinate with their vibrant, ever-changing play of color, a unique phenomenon in gemology. This phenomenon is not caused by pigments or chemical impurities, but by a physical interaction between light and the stone's internal structure. This article explores in detail the interference process responsible for this.
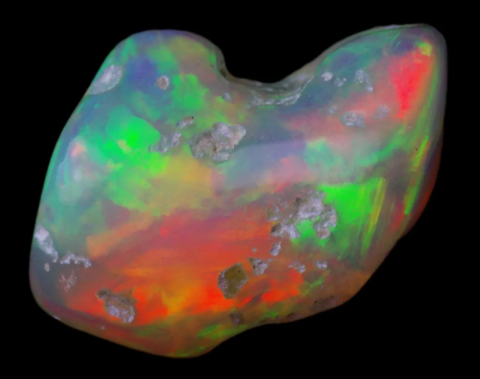
Photo : Opale from Welo, Ethiopia © Rémi Bornet
The structure of precious opals
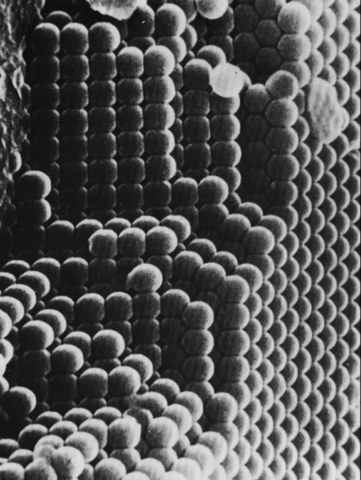
Opal is an amorphous form of hydrated silica (SiO2 nH2O). Unlike quartz, it does not have an ordered crystalline structure. However, some precious opals have an extremely regular arrangement of tiny silica spheres, organized into three-dimensional lattices and ranging in diameter from about 150 to 300 nanometers. These spheres, all of almost identical size, are naturally deposited over time in cavities or fractures, forming regular stacks similar to crystal lattices. This three-dimensional arrangement constitutes what is known as a photonic crystal, a structure in which the refractive index changes periodically. This type of lattice interacts with light in a particular way, allowing diffraction and interference of certain wavelengths. The alternation between silica spheres and interstitial spaces, often filled with water, generates a regular optical modulation capable of decomposing white light into distinct colors.
Photo : SEM image of organized silica spheres of a precious opal
The phenomenon of light interference
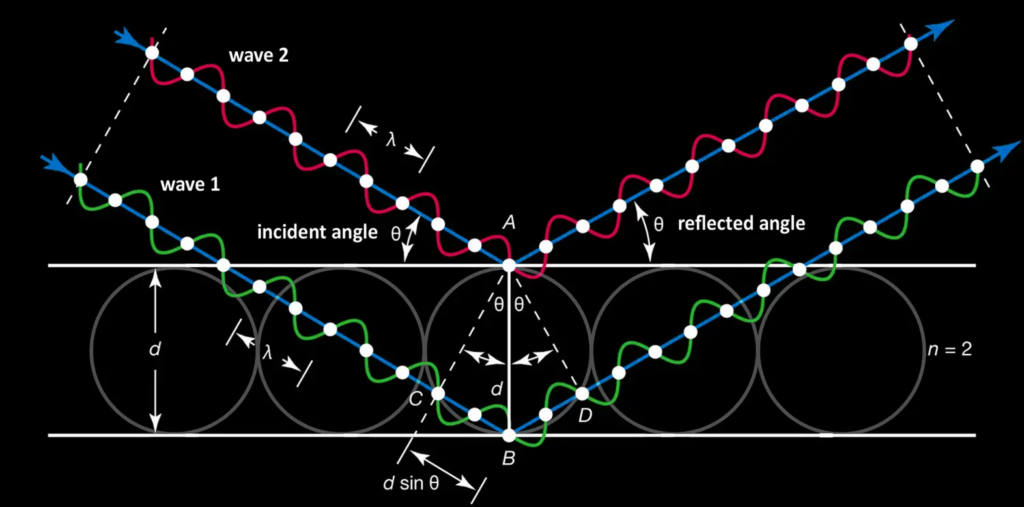
White light, which contains all visible colors, penetrates a precious opal and encounters its regular network of tiny silica spheres, neatly arranged like marbles stacked in layers. At each interface between these spheres and the thin layers of water separating them, some of the light is reflected, while another part continues its path through the structure.
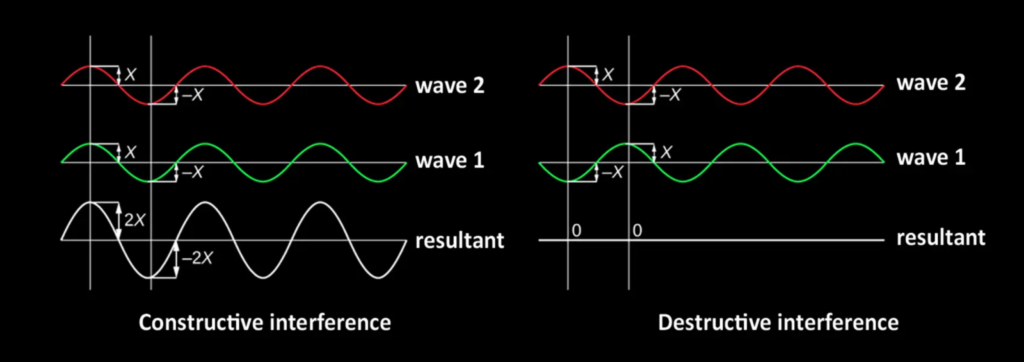
The reflected rays can interfere with each other depending on the difference in distance they have traveled within the stone. If this difference corresponds to an integer multiple of the wavelength (λ) of the light, the waves reinforce each other: this is constructive interference, which makes this color visible and brilliant to our eyes. If, on the other hand, they are out of phase (in phase opposition), they partially or completely cancel each other out; this is called destructive interference.
This phenomenon is described by Bragg's law : nλ = 2d sinθ
where :
- λ = wavelength of light (color)
- d = distance between the planes formed by the spheres
- θ = angle of incidence of the light
Diagram of constructive interference governing the play of color in precious opals.
Bragg's law derives from simple trigonometry by considering the reflection of light waves on planes of parallel silica spheres : the angle of incidence θ forms a right triangle with the spacing d between the planes.
Exemple :
Let's take a concrete example : suppose the silica spheres have a diameter of 300 nanometers (nm). This means that the distance between the planes is approximately 300 nm. If the light arrives at a particular angle, say 30°, the enhanced wavelength (according to Bragg's law) can be calculated :
λ ≈ 2 × 300 nm × sin 30° ≈ 2 × 300 nm × 0.5 ≈ 300 nm.
300 nm corresponds to ultraviolet, which the human eye cannot see. But by slightly changing the angle (for example, by moving the stone), or by taking into account higher-order interference (n = 2, 3, etc...), we can reach visible wavelengths such as 600 nm (orange-red) or 450 nm (blue).
In short, every time you tilt or turn the opal, you change the angle θ, which modifies the visible colors. This is what creates the shifting and changing play of colors characteristic of precious opals : a marvel of light diffraction revealed by a perfectly natural microscopic organization.
Size of spheres and observed colors
The observed color depends directly on the diameter of the silica spheres. The smaller these spheres are, the more they promote the diffraction of short wavelengths, which corresponds to blue or violet hues. When the spheres have an intermediate diameter, it is more greens and yellows that appear, while larger spheres allow the diffraction of reds and oranges. We can therefore understand why opals with red flashes are considered the rarest and most valuable. Conversely, an opal whose spheres are too small or poorly arranged will offer a limited spectrum, dominated by blues or greens, or even no play of colors if the structure is too disorganized. The intensity and diversity of colors are therefore directly linked to the size, homogeneity and regularity of the network of spheres.
Natural comparisons : photonic structures elsewhere
This phenomenon of coloration by periodic structure, called structural coloration, is found in other wonders of nature. The wings of the morpho butterfly, for example, have keratin microstructures arranged in regular layers that cause light interference similar to that of opals, producing a brilliant blue without any pigment. Peacock feathers are also structured to reflect certain wavelengths depending on the viewing angle. In the marine world, the nacre lining the inside of shells, such as those of abalone or giant clams, is formed of thin alternating layers of calcium carbonate and organic matter. These regular stacks create an iridescent shimmer resulting from multiple interferences. In all these cases, the color is produced by the physics of the surfaces and not by the chemistry of the materials. This underlines how opals share a fascinating kinship with other natural systems where light is sculpted by matter to produce spectacular visual effects.
Common Opals vs. Precious Opals
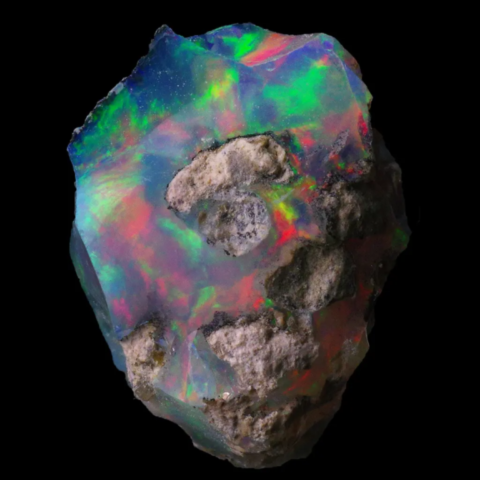
It is essential to distinguish precious opals, which exhibit an intense play of color, from so-called common opals, which lack it. Although their chemical composition is identical, common opals lack this orderly organization of spheres on a nanoscale. Their spheres vary in size and are randomly distributed, preventing any coherent diffraction of light. In the absence of a periodic structure, there is no constructive interference and therefore no vibrant colors. Visually, these common opals appear opaque, milky, or simply translucent, without the typical iridescent effect. This clearly shows that the phenomenon is not a matter of substance, but of microscopic architecture. The arrangement of the spheres is the key to the light spectacle offered by precious opals.
Synthetic and imitation opals
Humans have successfully reproduced the play of color in opals in the laboratory by creating synthetic opals. These are obtained by pressure deposition of calibrated silica spheres that faithfully imitate the natural arrangement. Synthetic opals can be very convincing, but they often have distinctive characteristics. The spheres are generally perfectly regular and arranged without defects, resulting in geometric patterns that are sometimes too perfect. The play of color can then appear too uniform or exhibit a typical snakeskin texture under magnification, or even columnar. As for plastic or glass imitations, they have only a deceptive superficial appearance without an ordered internal structure and produce optical effects lacking depth. Although used in jewelry for their lower cost, they do not possess the optical richness or value of genuine natural opals.
Conclusion
The play-of-color for which precious opals are renowned results from a physical phenomenon of light interference produced by the regular arrangement of silica spheres on a nanometric scale. White light is diffracted and recombined according to precise laws, revealing a range of colors that change with the slightest movement. The opal thus becomes a remarkable example of the beauty born of the natural order, where science and aesthetics come together to create a stone that is both mysterious and rich in color.